WillD
Expert
- Joined
- Jul 19, 2021
- Messages
- 645
- Reaction score
- 895
- Points
- 93
Reaction scheme:
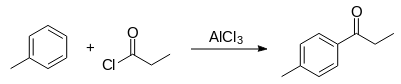
Reagents:
Propionyl chloride - 1000 g,
Toluene - 3000 ml,
AlCl3 anhydrous - 1500 g.
Equipment:
Flask,
Reflux condenser,
Drip funnel,
Distiller (straight condenser),
Mechanical stirrer.
All parts of the device must be carefully dried. Toluene applies anhydrous and taken in excess, as it serves as a solvent.
Synthesis:
1. In flask, equipped with a reflux condenser, a calcium chloride drying tube, drip funnel and mechanical stirrer, place aluminum chloride and toluene.
2. Start stirring and was added dropwise propionyl chloride with such speed, to the uniform release of hydrogen chloride.
3. In the case of a rapid reaction, outer cooling is needed.
4. The end of the reaction is determined by the cessation of hydrogen chloride.
5. After completion of the reaction, the reaction mixture was poured onto crushed ice and add a diluted hydrochloric acid solution to dissolve the aluminum hydroxide precipitate.
6. The top, benzene layer separated from the lower, water layer.
7. From the aqueous layer extracted with 4-methylpropiophenone with a small amount of benzene.
8. Benzene solutions are condensed and washed with a dilute solution of sodium hydroxide, water and dried magnesium or sodium sulfate.
9. After the toluene is distilled off to get 4-methylpropiophenone (mp 239 *С).
Reagents:
Propionyl chloride - 1000 g,
Toluene - 3000 ml,
AlCl3 anhydrous - 1500 g.
Equipment:
Flask,
Reflux condenser,
Drip funnel,
Distiller (straight condenser),
Mechanical stirrer.
All parts of the device must be carefully dried. Toluene applies anhydrous and taken in excess, as it serves as a solvent.
Synthesis:
1. In flask, equipped with a reflux condenser, a calcium chloride drying tube, drip funnel and mechanical stirrer, place aluminum chloride and toluene.
2. Start stirring and was added dropwise propionyl chloride with such speed, to the uniform release of hydrogen chloride.
3. In the case of a rapid reaction, outer cooling is needed.
4. The end of the reaction is determined by the cessation of hydrogen chloride.
5. After completion of the reaction, the reaction mixture was poured onto crushed ice and add a diluted hydrochloric acid solution to dissolve the aluminum hydroxide precipitate.
6. The top, benzene layer separated from the lower, water layer.
7. From the aqueous layer extracted with 4-methylpropiophenone with a small amount of benzene.
8. Benzene solutions are condensed and washed with a dilute solution of sodium hydroxide, water and dried magnesium or sodium sulfate.
9. After the toluene is distilled off to get 4-methylpropiophenone (mp 239 *С).
Last edited by a moderator:
